Microneedle drug development is classified by the various technologies used in each API drug.Usually, even if we have already established a mature microneedle industrialization production line when encountering a specific drug, we still have to carry out a series of pharmaceutical research, furthermore follow-up production process research. Moreover, when the type of drug changes, the production process and hardware of microneedles also change.BIOQINGLAM systematically design a drug according to the final output result. The current team has staffed with complete personnel such as preparation development, preparation analysis, process research, quality research, equipment development, and regulations.
- Genetic drugs: :DNA and mRNA
- Biologics: peptides and monoclonal antibodies: peptides and monoclonal antibodies
- Water-soluble chemicals:
- Poorly water-soluble chemicals:
- Research and development of vaccines:
According to the technical characteristics of different types of APIs and microneedle dosage forms of pharmaceutical customers, APIs can be classified into biological and chemical drugs.
Most biological drugs are water-soluble, and generally prepared by needle tip loading process. Drug formulation is relatively simple, and more importantly, through formulation design to control subsequent drug behaviour and output the expected R&D curve.
Biological drugs continue to be divided into two categories:
One is APIs acting directly, such drugs are direct-acting APIs that do not require further conversion in the body, such as monoclonal antibodies and peptides. The focus of microneedle preparations of such drugs is on stabilizing the preparation and accurately quantifying intradermal administration.
Genetic drugs include DNA and mRNA therapeutics, and in addition to the stability development of microneedle preparations, the activities of such drugs also required to be controlled in vivo through microneedle preparations, and we already have successful case of such drugs.
- CRO CASES
-
1. DNA vaccine microneedle preparations
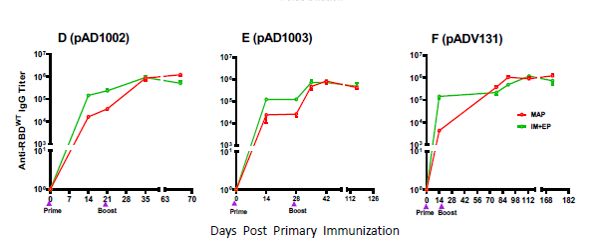
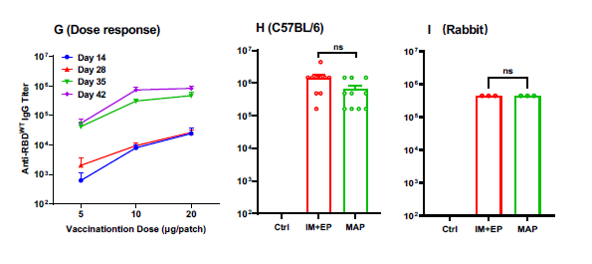
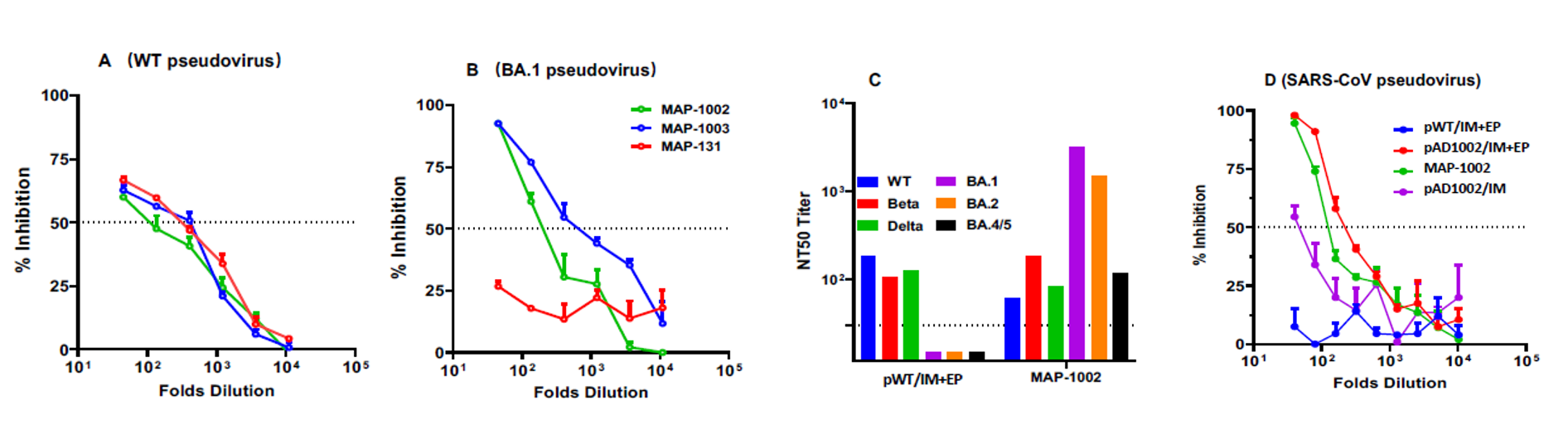
The project has successfully completed the small-scale, pilot-scale and pre-stability studies, and completed the corresponding animal experiments. In terms of clinical application, the project has achieved a huge breakthrough: compared to the combination of injection and electric shock, the microneedle patch can achieve the same effect in just 20 minutes of application. In addition, academic papers related to the research have been published in the international medical journal Emerging Microbes & Infections. https://doi.org/10.1080/22221751.2023.2202269 As an internationally renowned journal focusing on the field of emerging infections (impact factor: 19.568), the journal provides a fast, professional and efficient publishing platform for the latest scientific research results of the world's top scientists, and is one of the most timely and authoritative international journals for the study of the coronavirus.
- 2. GLP-1 analogue A
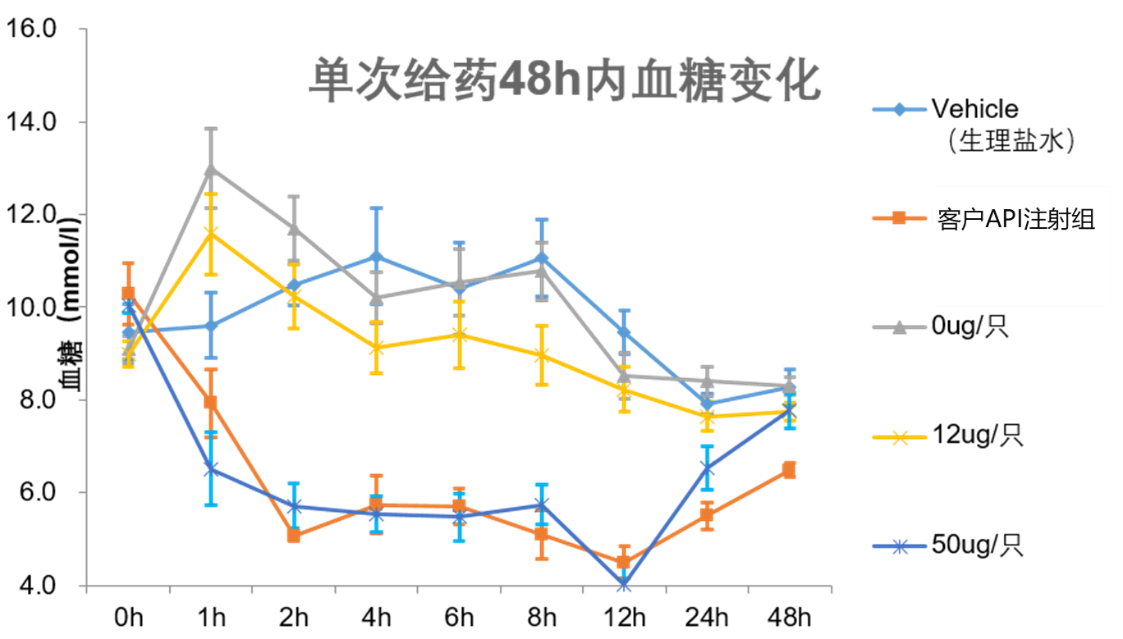
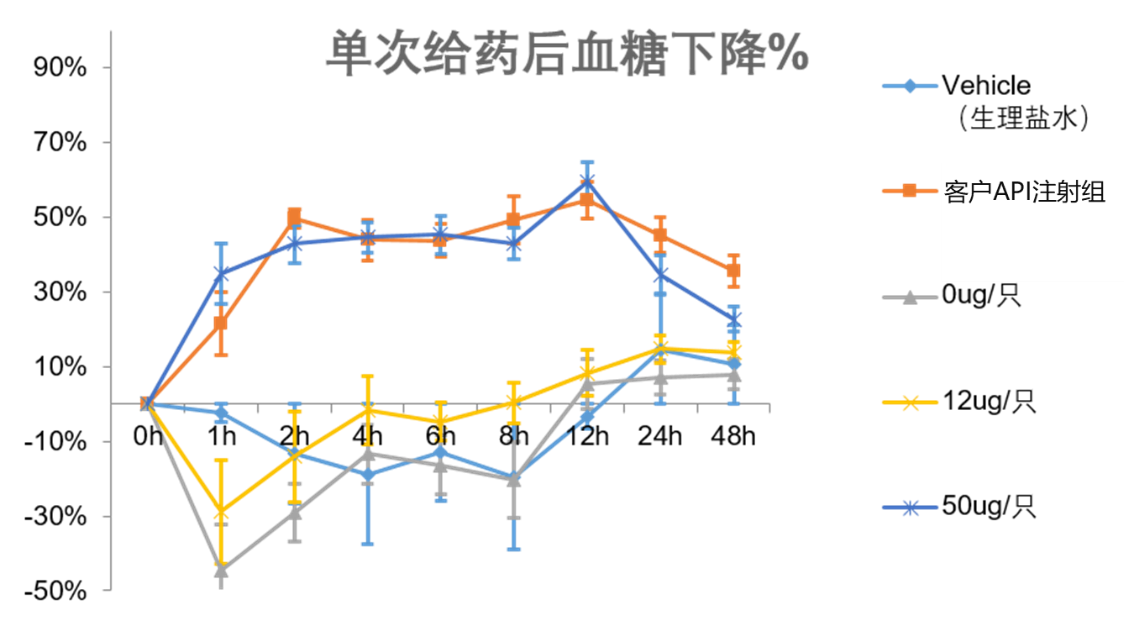
The project has now completed a feasibility study for pre-microneedle subcutaneous drug delivery with the customer. The results of the PD test showed that the soluble microneedle patch was comparable to the hypoglycaemic effect of db/db mice and C57 mice in the subcutaneous injection group. The preliminary test sample has good stability at room temperature for two months, and the results of single and multiple administrations are stable and reproducible, which has been recognized by customers.
- 3. GLP-1 analogue B
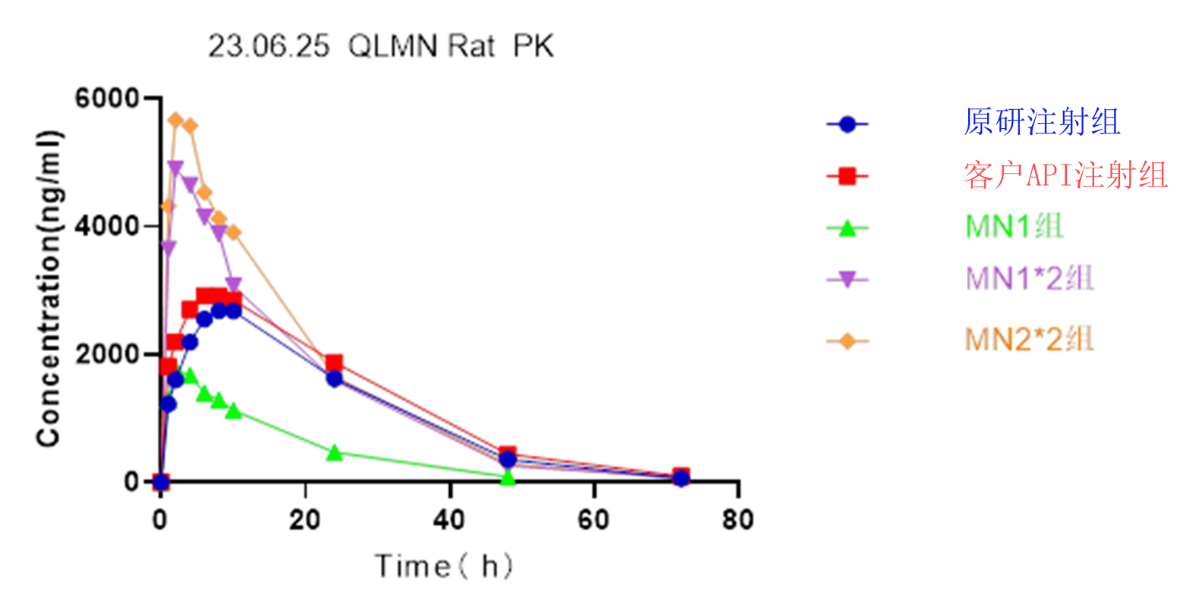
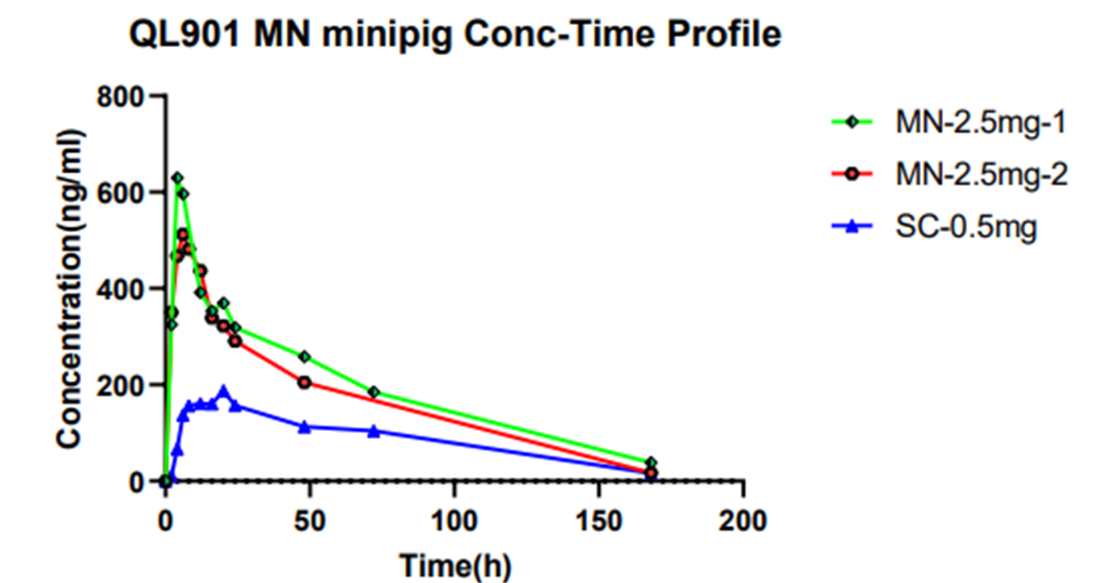
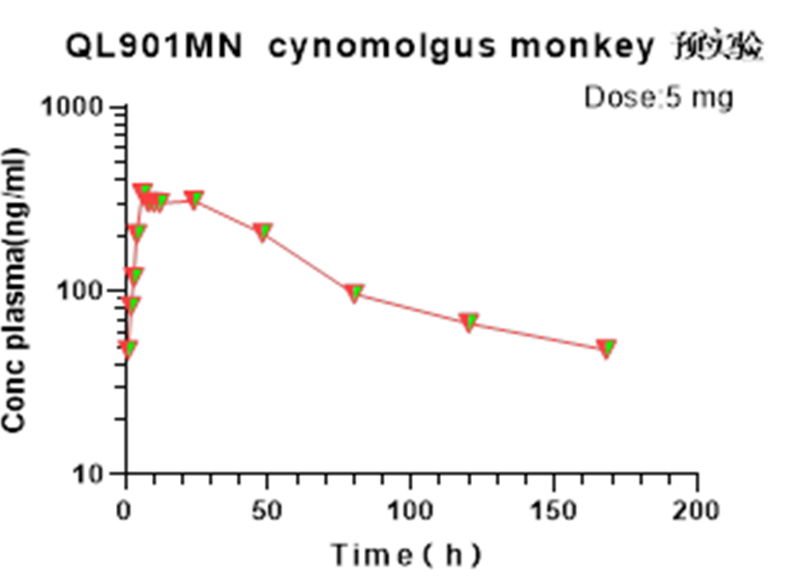
- 4. GLP-1 analogue C
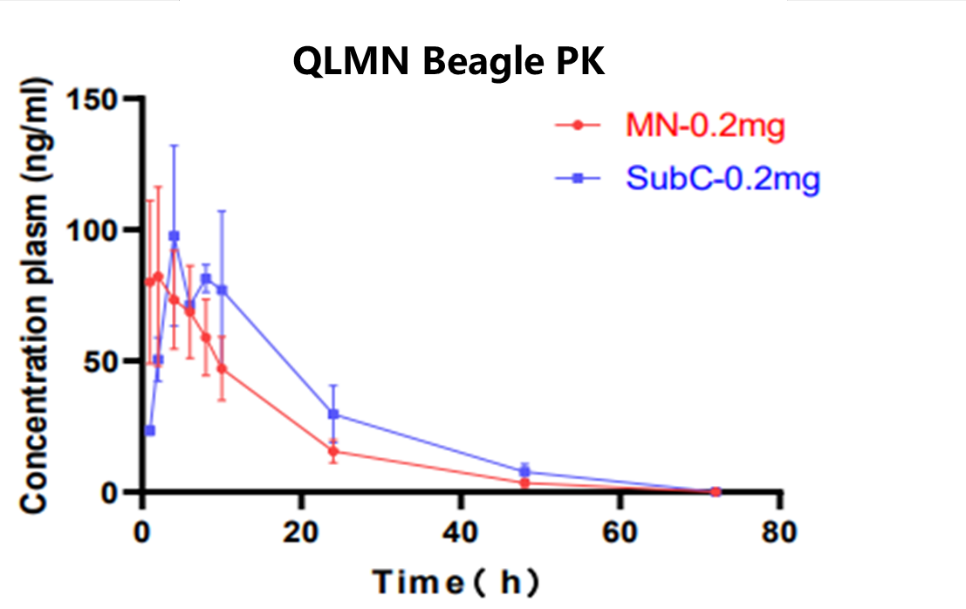
- 5. A chemical sedative drug
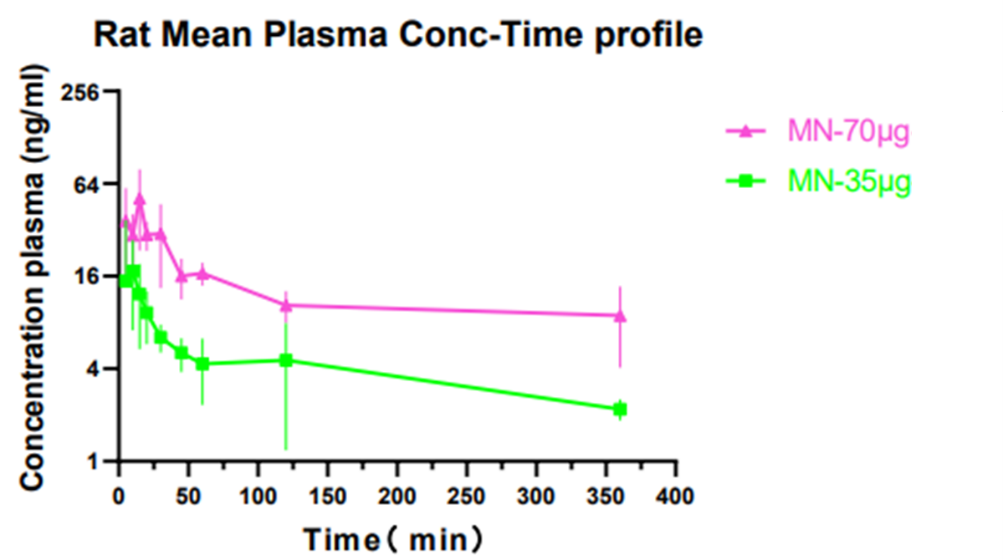
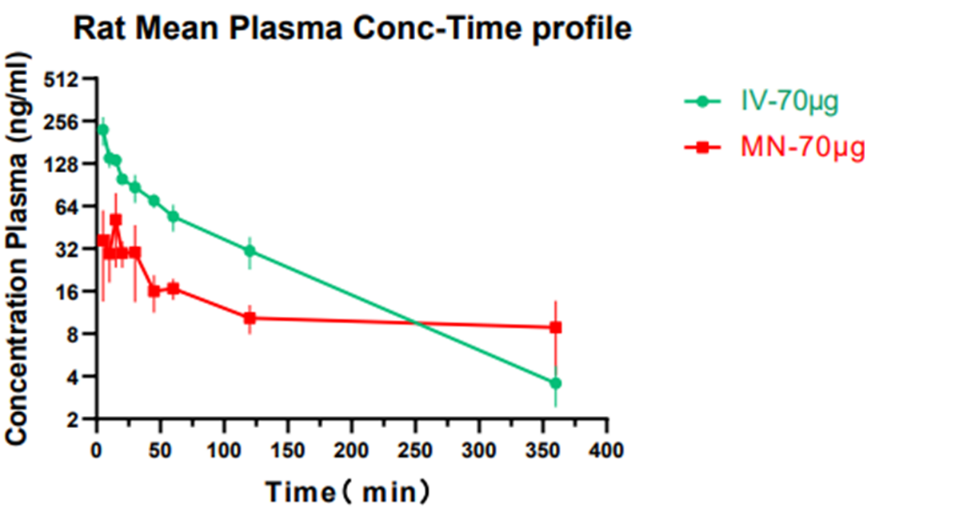
Microneedle drug development is classified by the various technologies used in each API drug.Usually, even if we have already established a mature microneedle industrialization production line when encountering a specific drug, we still have to carry out a series of pharmaceutical research, furthermore follow-up production process research. Moreover, when the type of drug changes, the production process and hardware of microneedles also change.BIOQINGLAM systematically design a drug according to the final output result. The current BIOQINGLAM team has staffed with complete personnel such as preparation development, preparation analysis, process research, quality research, equipment development, and regulations.





